Cosmetics and DGSA Specialist
Phone +358 10 504 5860
satu.salomaki@chementors.eu
Finnish | English
We offer tailored solutions for updating and revising your safety data sheets (SDS, MSDS) based on country-specific regulations and hazard classifications.
We can update and revise your SDSs for all industries and target market areas.
Contact us now!
Chementors handles regulatory updates related to hazard classification and country-specific requirements for safety data sheets, ensuring full compliance for clients.
At Chementors, safety data sheet updating is our daily basis task. We have the knowledge and experience of over 12 years in SDS legislation systems around the world which enables our team to keep up with revisions in SDS requirements and hazard classification of substances or mixtures.
We take care of regulatory updates that are particularly relevant to clients to inform and act in a timely manner.
At each stage of the process, we keep checking and evaluating the details and wholeness of the SDS to ensure the comprehensiveness and compliance of the ready-to-go document.
In addition, our SDS authoring software – Chemeter – regularly and automatically updates the hazard classification of substances, OEL values, toxicological data, and SDS format and content requirements of different countries. Its functions also enable us to revise changes in product formulation and input new data on physicochemical properties in a fast and easy way.
A safety data sheet (SDS) is considered as a “living” document in which the intrinsic hazard information, risk management measures, and regulatory requirements could be varied over time. It is a good practice to update SDS regularly, for example, every year or every two years. In cases where SDS updating is legally compulsory, the SDS must be updated without undue delay once it meets the criteria specified by the regulation. The SDS document needs to be updated frequently to:
Under the scope of certain legislations, it is a legal obligation to update SDSs without delay.
Our aim is to handle all SDS updating requirements for clients, ensuring that the current version of the safety data sheet is fully updated and in compliance with the regulations of the destination markets.
Chementors will ensure smooth trading activities of products into and inside the country/region of interest by avoiding penalties due to non-compliance. By keeping SDSs updated, clients would also be assured that their target audiences are always provided with proper information on chemical hazards and risk management.




Phone +358 10 504 5860
satu.salomaki@chementors.eu
Finnish | English

Phone +84 39 6169 628
mi.nguyen@chementors.eu
English | Vietnamese

Phone | +358 9 2316 7090
antti.aalto@chementors.eu
Finnish | English
If your current SDSs have one of the following points, it is an indicator that the SDS is old and requires to be updated.
The SDS was composed based on old versions of the local regulations: check section 15 of the SDS
The SDS does not follow the 16-section format
The SDS has outdated hazard pictograms, hazard classes, warning words, and/or hazard phrases: check sections 2 and 3 of the SDS
Example: all hazard phrases i.e., R-statements such as R1, R2, R3, etc., were obsoleted and have been replaced by H statements.
All precautionary statements i.e., S-statements such as S1, S2, S3, etc., were obsoleted and have been replaced by P statements.
Examples of outdated and updated hazard pictograms



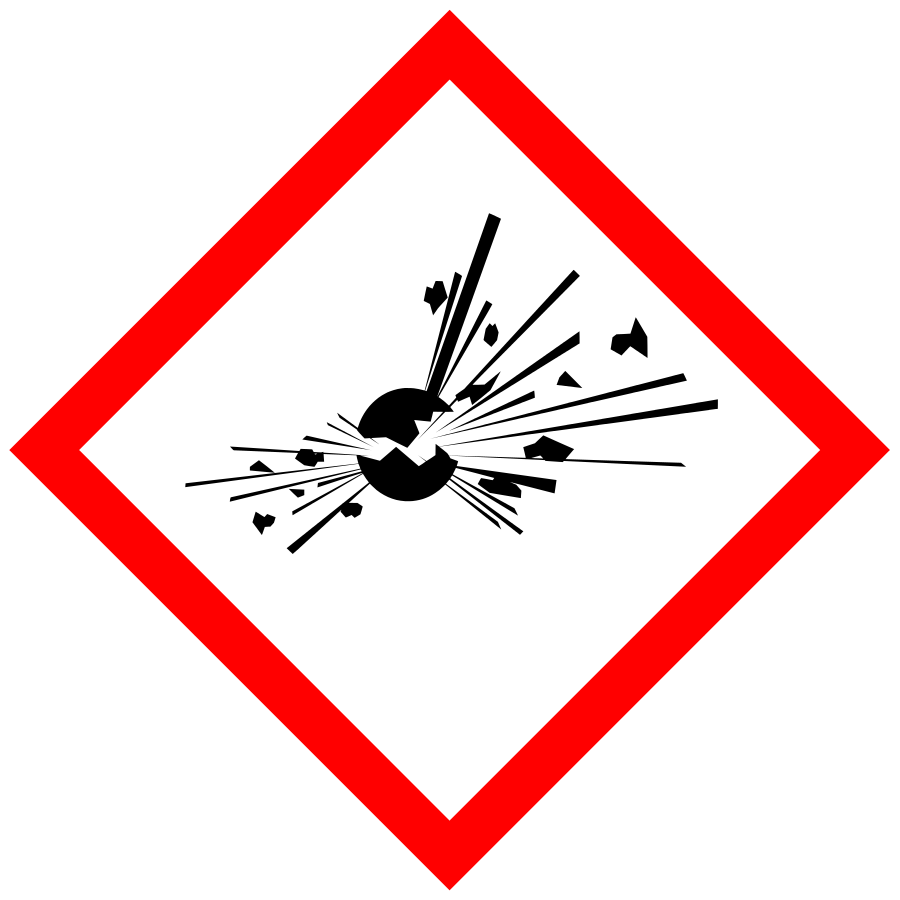

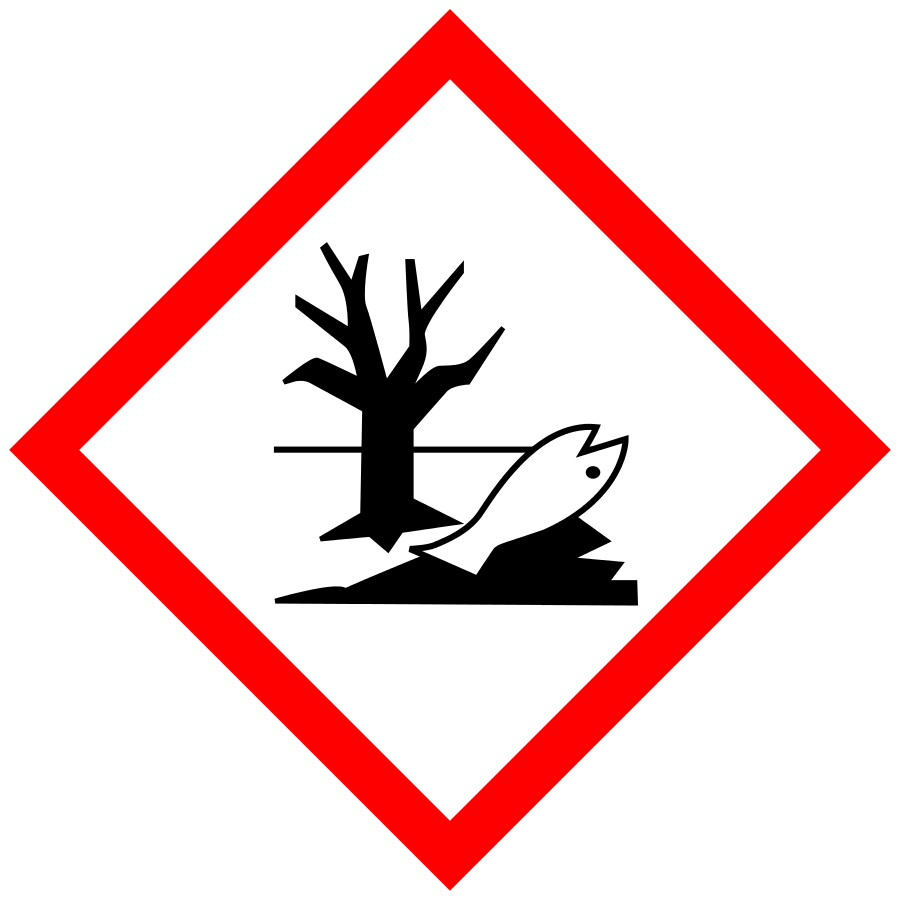






The SDS has outdated UN classes of dangerous goods for transport: check section 14 of the SDS
The SDS was composed long ago (for example 3 years ago)
Based upon newly developed scientific data and evaluations from competent authorities, countries often revise their legislations on hazard classification and labeling criteria, SDS format and content, occupational exposure limit (OEL) values, or special control rules and measures, etc., for certain chemicals. Therefore, manufacturers, importers, or relevant actors in the supply chain should check and update their SDS to guarantee that the latest SDS version well aligns with the current legislation of the destination markets. This compliance would help to smooth the movement of the chemicals into and inside the country.
In the EU, for example, the list of harmonized classification and labeling of hazardous substances is normally updated yearly by the European Commission following opinions from the Committee for Risk Assessment. Likewise, the candidate list of substances of very high concern (SVHC) for authorization is also regularly revised based on the proposal of the Member States or the European Chemicals Agency, with the purpose of gradually replacing highly concerned chemicals with less hazardous substances or technologies when it is plausible. SDSs of substances that fall into those lists should, therefore, be updated accordingly.
Under certain regulations, SDS revision is a legal obligation. For instance, Article 31 (9) of REACH lists specific occasions where SDS is required to be updated and reissued. Furthermore, it is compulsory to provide the updated SDS to every recipient in the supply chain to whom the substance or mixture has been supplied within the preceding 12 months.
Conditions in which suppliers are obligated to update SDS in accordance to Article 31 (9) of REACH:
(a) as soon as new information which may affect the risk management measures, or new information on hazards becomes available;
(b) once an authorisation has been granted or refused;
(c) once a restriction has been imposed.
There is no fixed list of information or documents that should be provided to have the SDS updated. Generally, it is very helpful to gather all available data, when possible, to make sure that the document is not only legally binding but also critical contents are covered in the SDS.
When it is possible and available, it would be useful for us to get the following information:
In cases where SDS updating is legally compulsory, the SDS must be updated without undue delay once it meets the criteria specified by the regulation. For substances that are subjected to EU-REACH, it is important to check if they fall into the scope of Article 31(9) of REACH about SDS updating obligation. For example, when a substance is included in the candidate list of SVHC, the current safety data sheet shall be updated in section 15 to show the identification of the substance as an SVHC.
For other cases, it would be a good practice to update SDS regularly, for example, every year or every two years. This step would make sure that the current version is fully compliant with local regulations, as countries and territories frequently issue revisions to their regulations.
Furthermore, it is also welcomed to voluntarily update the safety data sheet when there is new, additional, or revised information, which you think would be significant for target audiences during the handling, transport, and use of chemicals.




Chementors Ltd | Finland | Hong Kong | Vietnam | info@chementors.fi